IgM/IgG Antibody Rapid Test
Coronavirus disease( COVID-19) IgM/IgG Antibody Rapid Test
(Colloidal Gold)
Read the package insert carefully and completely before performing the assay. Follow the instructions and do not
modify them. Only by strict adherence to these instructions, the erroneous results can be avoided and the optimal
performance of Hotgen Coronavirus disease( COVID-19) IgM/IgG Antibody Rapid Test(Colloidal Gold) achieved.
INTENDED USE
This kit is used for in-vitro qualitative determination of Novel coronavirus(COVID-2019) Antibody in human serum
or plasma or whole blood. The Coronaviruses are single-stranded positive-strand RNA viruses, contain four genera:
α, β, γ, and δ; the neovel coronavirus (COVID-2019) is a new type of coronavirus, was found for the first time due to
Wuhan virus pneumonia cases in 2019 , belonging to the genus β and classified as a seventh coronavirus. Its genetic
characteristics are significantly different from SARS-CoV and MERS-CoV. On February 11, 2020, it was named
novel coronavirus (COVID-2019), which can cause viral pneumonia. The main clinical symptoms are fever and
fatigue. The respiratory symptoms are mainly dry cough and gradually have difficulty in breathing. In severe cases,
acute respiratory distress syndrome, septic shock, and metabolic acidosis which is difficult to correct and
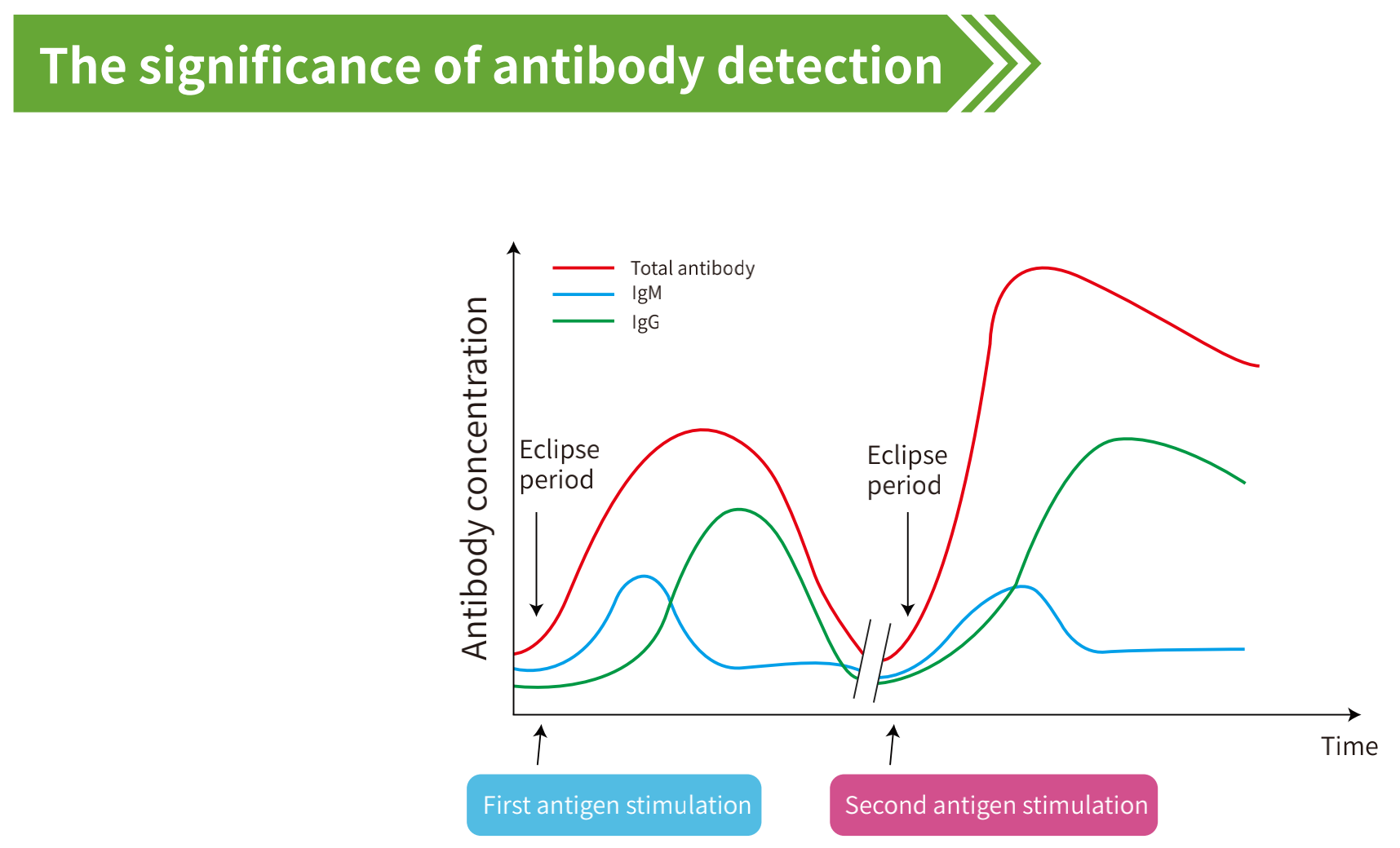
coagulopathy. The novel coronavirus (COVID-2019) IgM/IgG antibody is the antibody which is produced and
secreted by humans after being infected with the novel coronavirus (COVID-2019). IgM occurs earlier than IgG
antibodies and mainly exists in the blood. It increases with the development of the disease. After effective treatment,
IgM antibodies gradually disappear, while IgG antibodies persist. Novel cronavirus (COVID-2019) IgM/IgG
antibody test can be used to assist diagnosis of patients with novel coronavirus (COVID-2019) infection.
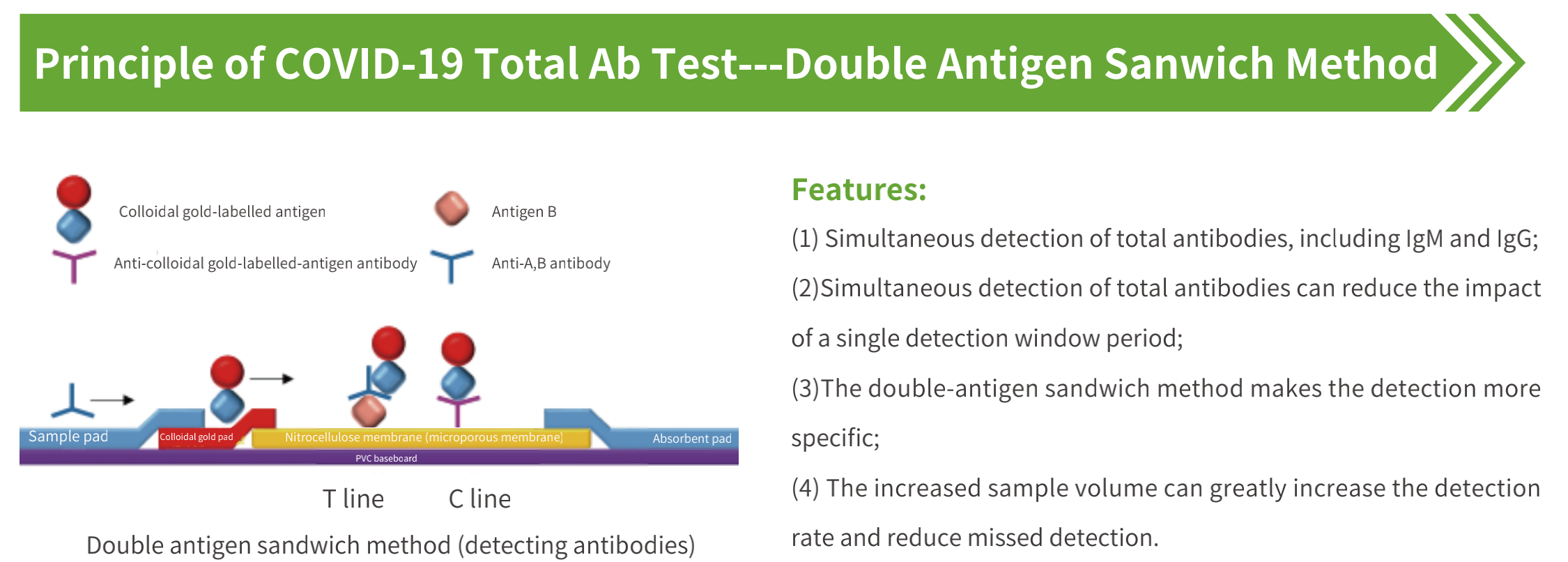
PRINCIPLE OF THE ASSAY
This kit is based on the colloidal gold immunochromatographic technology, and uses double antigen sandwich
method to detect the novel coronavirus IgM / IgG antibody in blood samples. The detection line (T line) of the novel
coronavirus IgM / IgG antibody test cassette was coated with CoV recombinant antigen, and the quality control line
(C line) was coated with goat polyclonal IgG. During the test, the sample is dropped into the test cassette and the
liquid is chromatographed upward under the capillary effect. The novel coronavirus IgM / IgG antibody in the
sample first binds to the colloidal gold-labelled CoV recombinant antigen to form a solid phase CoV antigen-CoV
IgM / IgG antibody-labelled CoV recombinant antigen-colloidal gold complex, at the C line position form a solid
phase goat polyclonal IgG-labelled CoV recombinant antigen-colloidal gold complex; after the test is completed,
observe the Colloidal gold color reaction of T line and C line to determine results of novel coronavirus IgM / IgG
antibodies in blood samples.
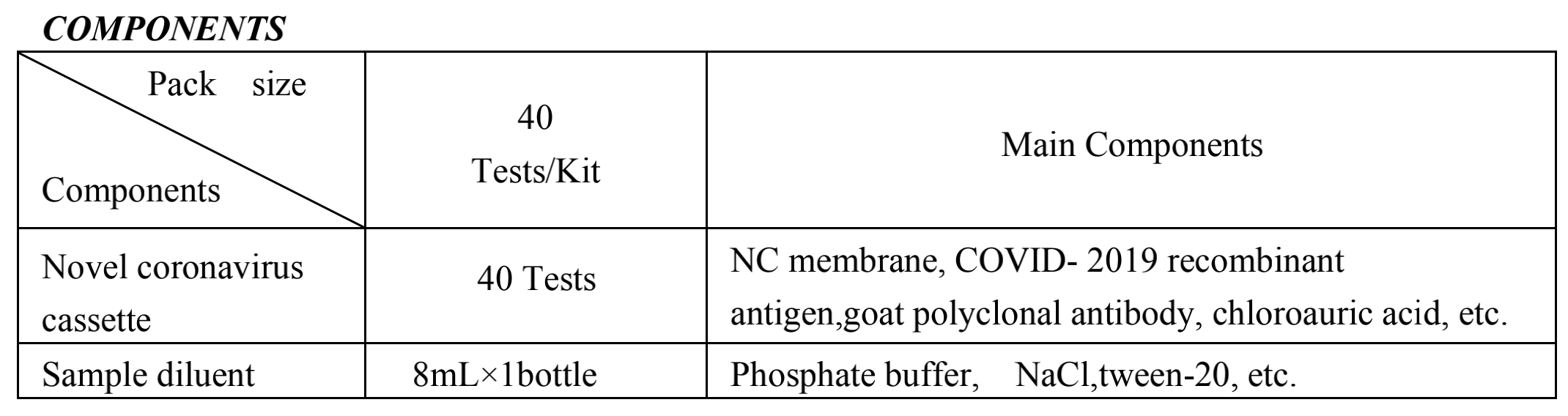
STORAGE AND VALIDITY
The kit should be stored at 4 ~ 30°C, the validity is set for 18 months.
See manufacturing date and expiration date on label.
SPECIMEN REQUIREMENT
Suitable for serum, plasma using heparin, EDTA or sodium citrate as anticoagulants and whole blood.
Serum and plasma samples should not be stored for more than 1 week at 4℃. If the detection cannot be performed
within 1 week after blood collection, the samples should be sealed and stored below -20 ℃ for less than 2 months.
Repeated freeze-thaw should be avoided, the number of times should not exceed 5 times. The thawed frozen samples
should be fully equilibrated to room temperature before being used for testing. The whole blood samples should be
tested within 8 hours after collection, and the whole blood samples should be tested immediately after
collection;Severe hemolytic and lipemia samples shouldn’t be used for testing.
TEST PROCEDURE
1. Place the test cassette, diluent and test sample at room temperature for 15-30 minutes, and equilibrate to room
temperature (20-25℃).
2. Open the aluminum foil pouch of the test cassette, and place the test cassette on a flat surface.
3. Write sample ID on the plastic case of the test cassette.
4. Serum or plasma: Take 10 μL sample, add it into the sample well of the test cassette, and add 3 drops of sample
diluent.
Whole blood: Take 25 μL (add 3 drops with a dropper) sample, add it to the sample well of the test card, and add 3
drops of sample diluent.
5. Incubate for 15 minutes at room temperature. Result got after 30minutes is invalid.
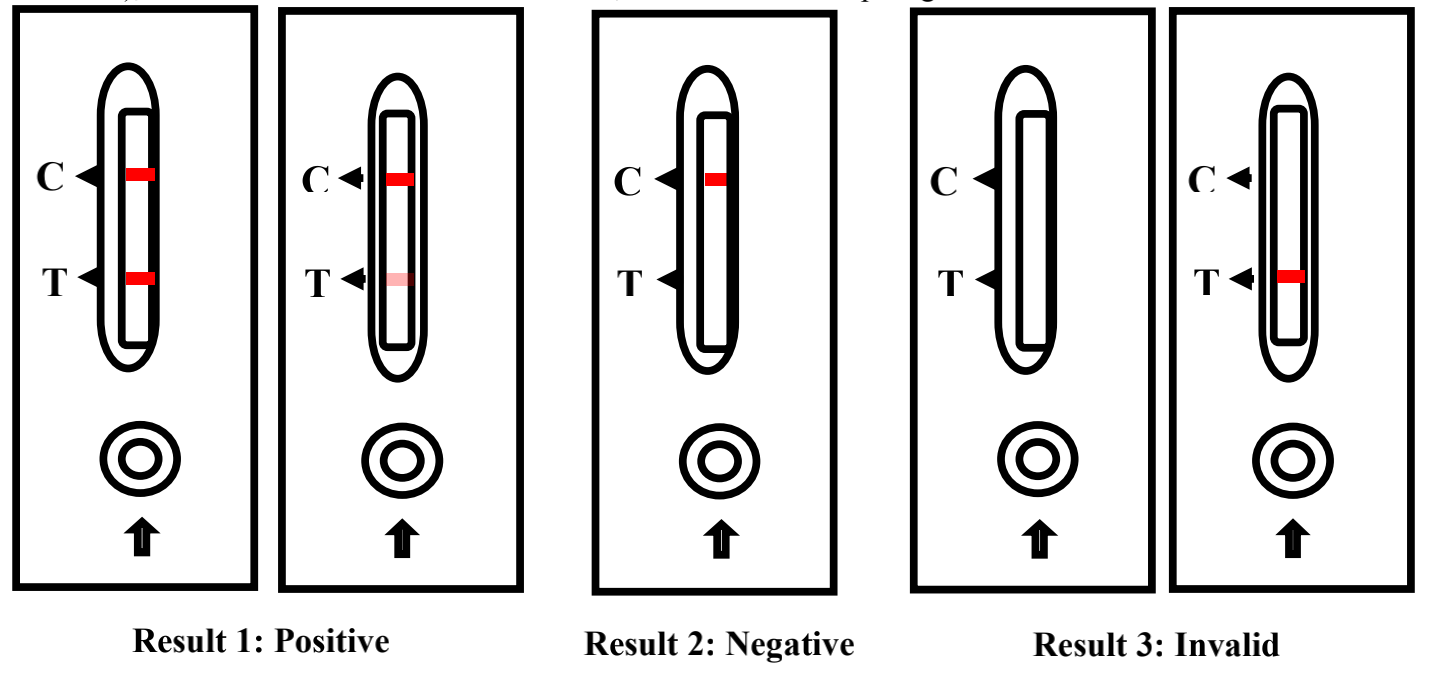
INTERPRETATION OF RESULT
Positive: Two color bands appear in the observation window, that is, a red or magenta line appears at the position of
the quality control line (C line) and the detection line (T line) (as shown in result 1), which indicates the test result of
novel coronavirus IgM / IgG antibody in the sample was positive.
Negative: A red or magenta line appears at the position of the quality control line (C line) in the observation window,
and no line appears at the position of the test line (T line) (as shown in the result 2), indicating the test results of the
novel coronavirus IgM/IgG in the sample were negative or the concentration was below the limit of detection of the
kit.
Invalid: No line appears in the position of the quality control line (line C) in the observation window (as shown in
result 3), which indicates that the test is invalid, should collect sample again and retest.